Electric Vehicle Series (Part 5)
It’s finally time to see how we can resolve the problem of battery pollution! In this article, we’ll be going over current solutions and relatively new technologies. We’re going to start off with how batteries are recycled, and slowly move on to the introduction of silicon carbon, a new material and a new mixture to enforce anodes, before moving on to the next step of using silicon for whole anodes and cathodes itself for more effective use. Without further ado, let’s get to the fun part and the main bulk, these buzzing solutions!
Reduce, reuse, recycle is a popular saying referring to how we should find new uses of a product to avoid pollution instead of throwing things away where they’ll end up in the landfill or ocean. This can also be applied towards electric vehicle batteries. Electric batteries still have up to 80% of their charge left when they are deemed unworthy to be used in cars. That’s still plenty of energy, so before batteries go through the recycling process, they are used in other places in a greater need of electricity, namely where solar panels and windmills are used. As energy efficient as these contraptions are, weather is very unpredictable, so the presence of the batteries help to make sure the people living there have enough electricity.
Other than that, there are two seperate ways to recycle batteries. If they are completely out of electric charge, then the battery is shredded. After the shredding, the various metal and copper pieces are sorted out to be reused. However, if there is still an electric charge in the battery, then it is very dangerous to handle it. It could explode in your face! In order to avoid that, it is frozen in liquid nitrogen. Liquid nitrogen is a really cold element, turning into liquid form at just 77 kelvin. That’s -196.15℃! The nitrogen is so chilled, that the batteries can no longer react and give off energy. After this process, the battery is smashed with a giant hammer, (which sounds quite fun! The people who work there must have a great way to release stress) and then after that, just like in the first method, the metals are sorted out into different piles for reuse.
Great, we have a way to appropriately recycle and reuse batteries! But why stop there? Can we find a way to prolong the life and efficiency of an EV battery before it’s deemed unworthy?
Well, scientists at the Korea institute of Technology seem to have found the answer. They were able to tweak the anodes and cathodes that we discussed in the previous article in order to make the battery better. These researchers have created a kind of ‘soup’ to enforce them. By mixing potato starch into water and then combining it with a mixture of silicon and corn oil, they have created a substance called carbon silicon. This mixture, when poured into the batteries, does something extraordinary. Naturally, graphite doesn’t have a very large storage capacity, but by adding the carbon silicon, the battery gets a giant energy boost. Silicon itself has 10 times the storage space of just graphite as well as 4 times the charge capacity. Using this mixture, the battery gets charged up to 80% in just 5 minutes!
Recent studies show that graphite-made battery anodes and cathodes are now being made completely out of silicon. But even after that, the silicon carbon electric soup still proves handy for enforcing these anodes and cathodes. You’ll be able to see how in just a moment. But first, why does silicon make such a good pairing with lithium ions? If the molecules inside both substances are observed, then one can see that silicon can bond with lithium ions 25 times better then what graphite can do.
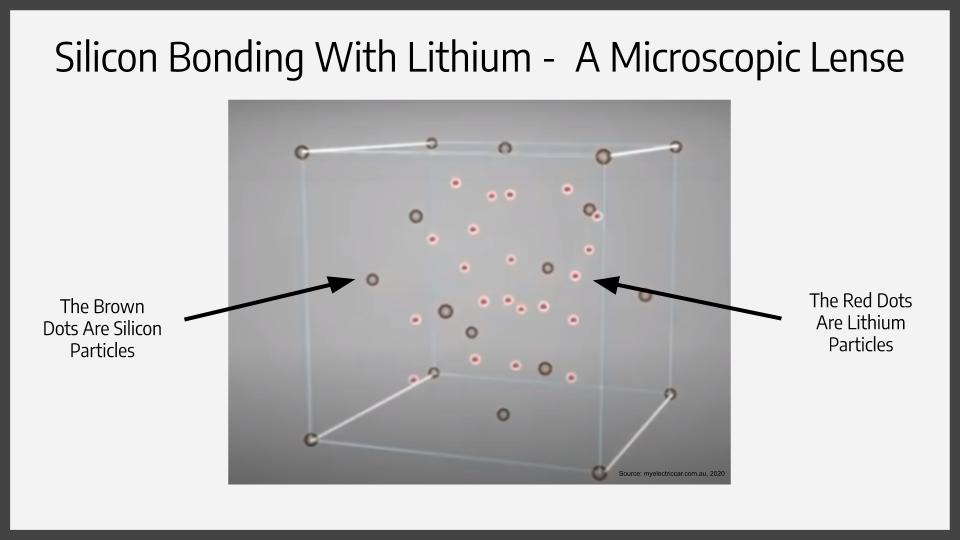
Now in all electronic devices, there is at least 1-5% silicon, with that number subject to change over time. This magical element is increasing battery density by 20-30% and upwards. The only con to the substance, being the reason it isn’t widely used in electric cars is that silicon cathodes and anodes bloat. When charging, the silicon anodes swell up, and when they lose charge, they become smaller. This repeating process breaks the silicon and causes unfixable damage to the battery overtime. However, that’s where the electric soup comes in to play. It allows the silicon to continuously bloat and contract without damaging the shell and causing it to deteriorate.
We’ve been through some of the current ways we are solving the problem of battery pollution, in finding ways to reuse them, recycle them, and even prolong their lifetime. But to stop here wouldn’t do justice to the entire topic of electric car batteries and what we can do for a better future. In the next article, we’re going to be looking closer at electric battery development & manufacturing companies, and taking a look at how different companies troubleshoot problems regarding their batteries. Stay tuned for a look at the inner working of some of the big companies, like Tesla and Samsung, and what new innovative technologies they’re bringing to the table.